As regenerative medicine and stem cell technologies continue to progress, so the list of tissues and organs that can be grown from scratch – and potentially replaced – continues to grow. In the past few years, researchers have used stem cells to grow windpipes, bladders, urethras and vaginas in the lab, and, in some cases, successfully transplanted them into patients.
Others are making progress in growing liver and heart tissue; one team in London is busy growing blood vessels, noses and ears; and some have even managed to grow tiny chunks of brain tissue, the most complex of all the tissues in the human body. Now, researchers in Germany report that they have grown complete spinal cords from embryonic stem cells.
Most efforts to grow tissues and organs rely on biodegradable scaffolds. When ‘seeded’ with a patient’s stem cells, these scaffolds provide a surface for the cells to latch on to and provide them with nutrients. The scaffold delivers the signals needed for the stem cells to differentiate along the correct path, and its structure coaxes them to form tissue of the right shape.
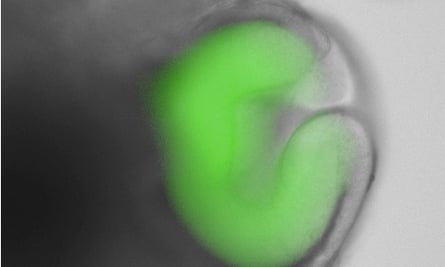
Nervous tissue is extremely complex, however. It starts off as a flat sheet of cells on the top surface of the embryo, called the neural plate, which, through a series of elaborate deformations, buckles and folds in on itself to form a hollow tube. One end of this neural tube will eventually form the brain, and the other the spinal cord. This complexity makes scaffolds unsuitable for growing nervous tissue, as they cannot be manufactured in the intricate shapes needed.
Andrea Meinhardt of the Dresden University of Technology and her colleagues therefore exploited a property of stem cells known as self-directed morphogenesis, first discovered by the late Yoshiki Sasai. About 10 years ago, Sasai and his colleagues developed a method for growing embryonic stem cells in three-dimensional suspension, and found that cells grown in this way can, when fed the right combination of signalling molecules, go through the motions of development and organize themselves to form complex tissues such as eyes, glands and bits of brain.
Meinhardt and her colleagues used a variation of Sasai’s technique, and embedded single-cell suspensions of mouse embryonic stem cells within a three-dimensional nutrient gel on Petri dishes. When left untreated, the cells begin to differentiate into immature neurons, giving rise to spherical structures containing immature cells resembling those found in the neural plate.
The spinal cord, like the brain, is a highly organized structure, with cells arranged in very specific patterns, along both the front-to-back and top-to-botton axes. In the embryo, a structure called the notochord acts as a signalling centre that guides the patterning of the spinal cord. The notochord forms beneath the neural tube and secretes retinoic acid, which activates a gene called sonic hedgehog in cells at the bottom of the neural tube.
These cells then synthesize and secrete the Sonic Hedgehog protein, which diffuses up through the tube, creating a ‘concentration gradient’. Immature neural tube cells are sensitive to the concentration of Sonic Hedgehog protein, and begin to differentiate according to their position in the top-to-bottom axis of the neural tube. Those nearest the bottom of the chord are exposed to the highest protein concentration, and this causes them to develop into the motor neurons that will later extend their fibres out to the muscles. Those further up are exposed to a lower concentration, and go on to form the interneurons that make up the local spinal circuitry.
Importantly, the embedded stem cells remained responsive to the signalling molecules they would be exposed to during development. Addition of retinoic acid to the gels therefore caused the cells to recapitulate development and organize themselves into spinal cord tissue with the various cell types arranged correctly. The researchers confirmed the identity of these cells by staining the lab-grown tissues with fluorescent antibodies that recognize and bind to different proteins synthesised only by each cell type.

Every year, hundreds of thousands of people around the world suffer partial or complete paralysis due to spinal cord injuries, but efforts to develop effective treatments have so far met with little success.
Last month, an Anglo-Polish research team announced that they had performed a novel cell transplantation procedure on a paralysed 38-year-old man, and that the procedure had led to some recovery of function in the patient’s legs. The long-term outcome of the procedure remains unclear, however, and its effectiveness has yet to be tested in randomised clinical trials.
This latest study will not immediately lead to new treatments, but the ability to grow patterned spinal cord tissue efficiently will undoubtedly prove useful to those who are trying to develop them. It could also provide researchers with a new way of studying spinal cord development, which could help them to learn more about congenital disorders such as spina bifida.
Reference: Meinhardt, A., et al. (2014). 3D Reconstitution of the Patterned Neural Tube from Embryonic Stem Cells. Stem Cell Reports, DOI: 10.1016/j.stemcr.2014.09.020.
Comments (…)
Sign in or create your Guardian account to join the discussion